Expanded Access (EA), also called Compassionate Use is a regulatory pathway that allows patients with serious or life-threatening diseases to access investigational drugs outside of clinical trials when no approved treatments exist. Unlike clinical trials designed to test safety and efficacy for regulatory approval, expanded access programs (EAPs) focus solely on providing treatment for patients with urgent unmet needs.
Expanded Access (also known as Compassionate Use) is a U.S. FDA and EU regulatory framework that enables patients to obtain investigational drugs when:
- No comparable or satisfactory therapy is available.
- The patient cannot join an ongoing clinical trial.
- The potential benefit outweighs potential risks.
Key Difference from Clinical Trials: Clinical trials generate safety and efficacy data under controlled conditions, while expanded access is strictly for treatment purposes, not research.
Right-to-Try legislation (2018) provides a separate pathway, allowing access without FDA oversight or IRB review, but requires that the drug has completed Phase I trials and that the manufacturer agrees.
Sponsors may offer EA when:
- Patients have serious or immediately life-threatening diseases.
- No standard or alternative treatment options exist.
- The patient cannot participate in available clinical trials.
- The potential benefit justifies potential risks.
EA pathways accommodate various development stages, though specific requirements vary by program type.
- Individual Patient Access: Generally requires Phase I safety data.
- Intermediate-size populations: Need stronger safety evidence.
- Treatment INDs (widespread use): Require substantial safety and efficacy data.
Sponsors must provide safety data showing:
- Known dose-limiting toxicities
- Possible drug–drug interactions
- Contraindications
The FDA evaluates whether “risks are not unreasonable given the disease severity.”
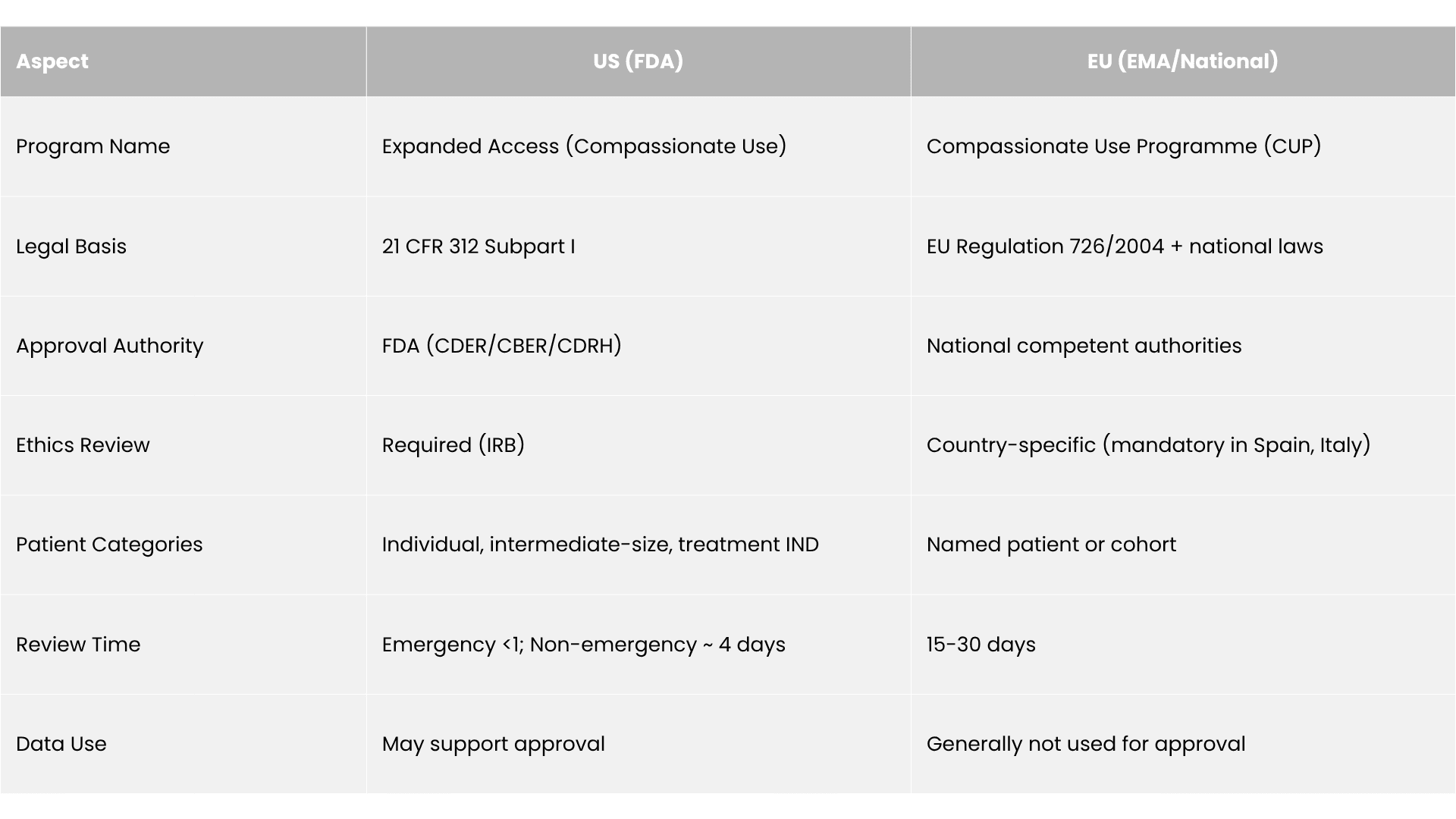
- United States: FDA authorization is required except Right-to-Try pathways. Emergency requests can be approved by phone in <24 hours.
- European Union: National competent authorities decide, with EMA offering coordination for multi-country programs.
Three main EA pathways exist under FDA:
- Individual patient INDs (including emergency use).
- Intermediate-size patient protocols.
- Treatment INDs for broad populations.
- Emergency use: FDA approval by phone; IRB notified within 5 days.
- Non-emergency: IND + IRB approval required; typical review time ~4 days.
European programs operate through national competent authorities under Article 83 of Regulation 726/2004. The EMA provides harmonized recommendations, but implementation varies by member state.
For example, countries like:
- France: Temporary Authorization for Use (ATU).
- UK: Early Access to Medicines Scheme (EAMS) with real-world data collection.
- US sponsors must maintain current INDs with protocol amendments for any changes, annual reports, and expedited safety reporting within 15 days of serious, unexpected, related adverse events.
- EU reporting requirements vary significantly by country, with some requiring periodic efficacy and safety updates.
- US: IRB approval is mandatory for all US EA programs except emergency use, where post-treatment notification within five days suffices.
- EU: Some countries (e.g., Italy, Spain) require ethics review; others delegate responsibility to physicians.
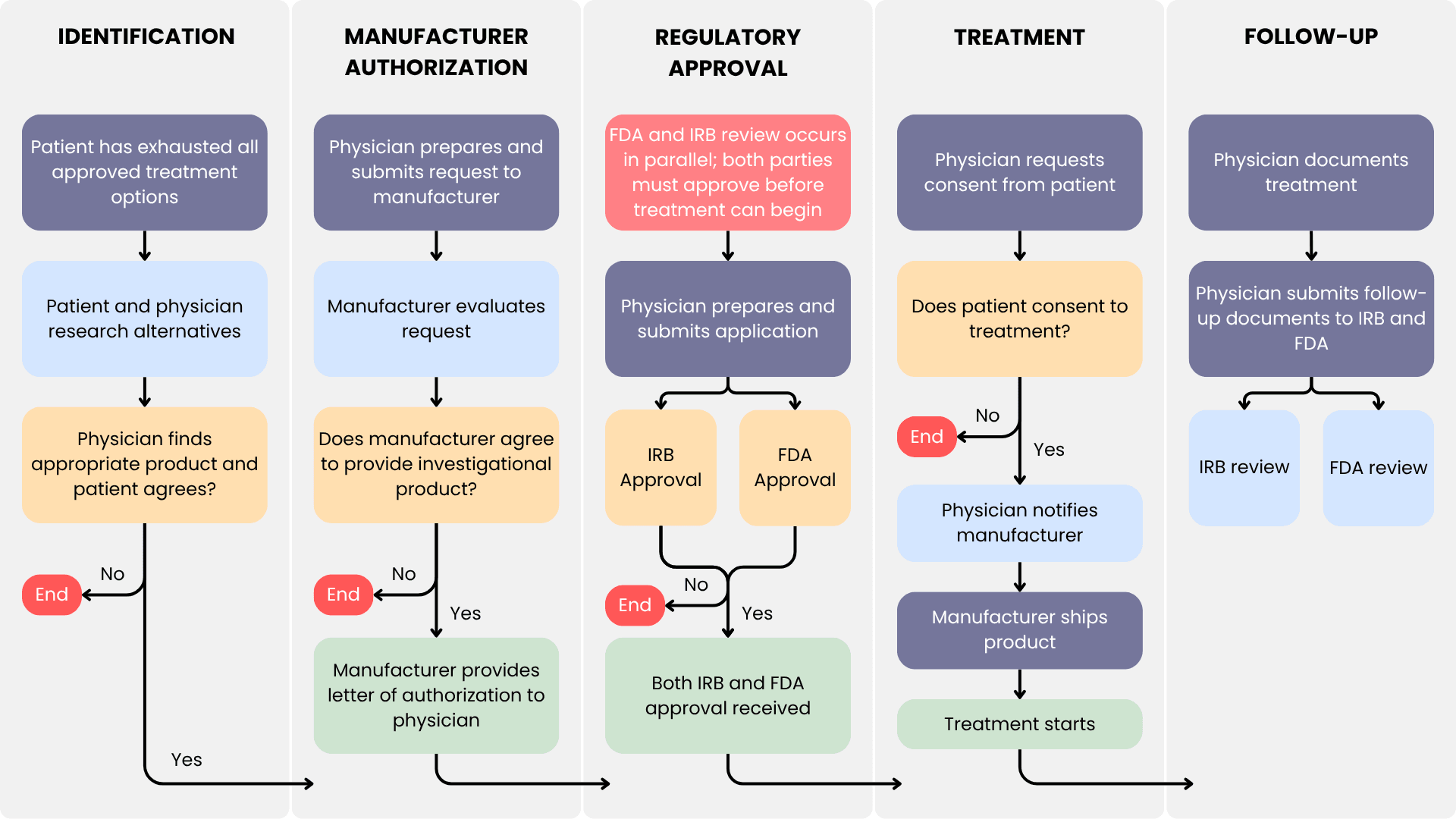
Expanded Access Request Process (Single Patient)
- Patient goodwill & reputation: EA programs demonstrate commitment to patient access and can enhance relationships with physician communities and patient advocacy groups.
- Real-world evidence: EA data increasingly used in regulatory submissions and health technology assessments (HTA).
- Regulatory advantage: FDA analysis shows drugs with EA have higher approval rates (84%) than those without (76%).
- Resource demands: Represents the primary operational challenges. Regulatory, medical, and supply chain teams must manage EA alongside trials.
- Manufacturing capacity: Small biotechs may struggle to supply both EA and trials.
- Regulatory obligations: These include ongoing safety monitoring, adverse event reporting, and protocol maintenance. However, FDA analysis of 321 regulatory decisions found no instances where EA experience led to negative approval decisions.
Managing Expanded Access requires precise coordination across regulatory submissions, patient eligibility tracking, safety reporting, and supply management. Many sponsors—especially emerging biopharma—struggle to balance these demands alongside pivotal clinical trials.
Zelthy’s digital health platform simplifies this process by:
- Streamlining regulatory workflows: Centralized tracking of IND submissions, IRB approvals, and country-specific documentation.
- Enabling real-time patient and physician engagement: Secure portals for request intake, eligibility screening, and consent management.
- Automating compliance reporting: Built-in tools for safety event capture, monitoring, and timely regulatory submissions.
- Scaling globally: Configurable modules that adapt to the U.S. FDA Expanded Access, EU Compassionate Use, and other country-specific frameworks.
By integrating operational, regulatory, and patient-facing functions into a single platform, Zelthy enables sponsors to expand patient access without straining resources—while maintaining compliance, transparency, and data integrity.
A leading Australian pharmaceutical company partnered with Zelthy to digitize its Compassionate Access Program (CAP), cutting approval times by 65%, reducing costs by 40%, achieving 99% compliance with automated audit trails, improving real-time visibility for stakeholders, and strengthening supply chain forecasting—ultimately delivering critical therapies faster and more efficiently.
See how Zelthy transformed compassionate access. Read the full case study here.
Book a demo and talk to us about building your Expanded Access framework with Zelthy. Contact us at connect@zelthy.com or send us a DM on LinkedIn.
- Office of the Commissioner. (2025, September 8). Expanded access. U.S. Food And Drug Administration.
- Jarow, J. P., Lurie, P., Ikenberry, S. C., & Lemery, S. (2017). Overview of FDA’s expanded access program for investigational drugs. Therapeutic Innovation & Regulatory Science, 51(2), 177–179. doi:10.1177/2168479017694850
- Ferdman, J. M., Peloquin, D., Ropes & Gray LLP, Bierer, B. E., & Multi-Regional Clinical Trials Center of Brigham & Women’s Hospital and Harvard. (2025). New developments in right to try legislation. [pdf]
- Klopfenstein, M., Van Campen, L. E., & Garnett, T. (2015). Expanded Access Programs: ethical and practical considerations for biopharmaceutical sponsors. Therapeutic Innovation & Regulatory Science, 49(3), 352–358. doi:10.1177/2168479015578154
- U.S. Food And Drug Administration (2017). Expanded Access to Investigational Drugs for Treatment Use — Questions and Answers Guidance for Industry. [pdf]
- European Medicines Agency (EMA) (2020). Compassionate use | European Medicines Agency (EMA).
- Whitfield, K., et al. (2010). Compassionate use of interventions: results of a European Clinical Research Infrastructures Network (ECRIN) survey of ten European countries. Trials, 11(1). doi:10.1186/1745-6215-11-104
- Holbein, M. E. B. (2009, August 1). Understanding FDA regulatory Requirements for investigational new Drug Applications for Sponsor-Investigators. [pmc]
- Polak, T. B., Van Rosmalen, J., & De Groot, C. a. U. –. (2020). Expanded Access as a source of real‐world data: An overview of FDA and EMA approvals. British Journal of Clinical Pharmacology, 86(9), 1819–1826. doi:10.1111/bcp.14284
- Wasser, J. S., & Greenblatt, D. J. (2023). Applying real-world data from expanded-access (“compassionate use”) patients to drug development. Journal of Clinical and Translational Science, 7(1). doi:10.1017/cts.2023.606
- Fountzilas, E., Said, R., & Tsimberidou, A. M. (2018). Expanded access to investigational drugs: balancing patient safety with potential therapeutic benefits. Expert Opinion on Investigational Drugs, 27(2), 155–162. doi:10.1080/13543784.2018.1430137
- Jarow, J. P., & Moscicki, R. (2017). Impact of expanded access on FDA regulatory action and product labeling. Therapeutic Innovation & Regulatory Science, 51(6), 787–789. doi:10.1177/2168479017707800
- Jarow, J. P., et al. (2016). Expanded Access of Investigational Drugs: The experience of the Center of Drug Evaluation and Research over a 10-Year period. Therapeutic Innovation & Regulatory Science, 50(6), 705–709. doi:10.1177/2168479016656030



